Elon Musk’s startup Neuralink has made a major move in the medical device world, achieving FDA approval to begin human clinical trials for its brain-computer interface technology. The technology could eventually be used to restore sight and mobility to people with disabilities, represent a major leap forward in restoring damaged neurological functions, and even bridge the gap between human and artificial intelligence.
While the technology has been developed since 2016 and went through the FDA approval process, it still is in the test phase. Last December, the company suggested that it would take another six months before beginning human trials. It appears that Neuralink was able to answer the FDA’s questions and was given the green light in a relatively short two month period.
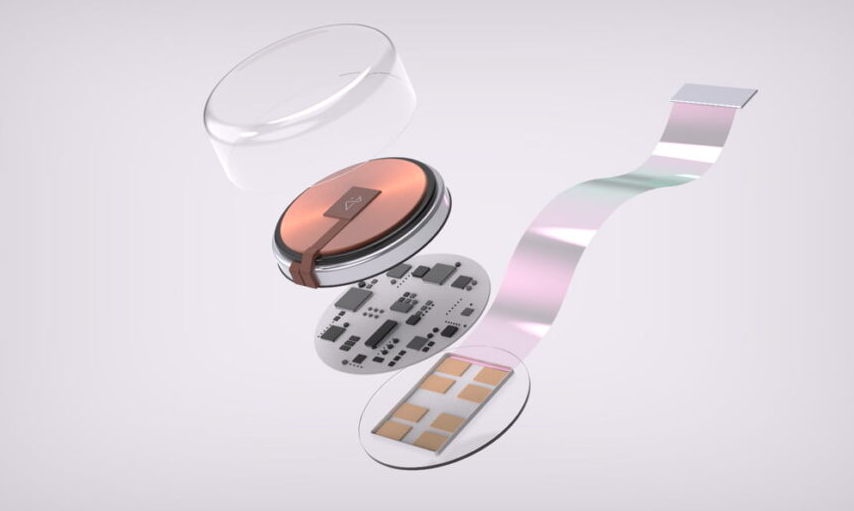
The chip called N1 is 8 millimeters in diameter and features wires that compare in size to neurons in the brain. This allows it to be implanted without touching any veins or arteries. Its capabilities include being able to control external devices with the mind, studying and treating neurological disorders, and even merging human intelligence with artificial intelligence to enhance cognitive abilities.
Neuralink is now in the process of recruiting people for the clinical trials. No other information has been made available as to what the trials will involve, but initial testing is expected to focus entirely on safety rather than efficacy. Neuralink’s surgical robot that performs the implanting also needs to gain FDA approval, so the full objectives of Neuralink have yet to be seen.
It is hoped that the clinical trials will demonstrate Neuralink’s potential to restore and enhance human brain functions, with pioneering implications for health care. This approval marks a major milestone in the development of Neuralink and could pave the way for a revolutionary new era of medical care.